Wonderful Tips About How To Teach Ionic Bonding

Magnesium loses two electrons and oxygen gains two electrons, leaving.
How to teach ionic bonding. This lesson sees to both through interactive simulations and clearly laid out slides. Worksheets and lesson ideas to challenge students aged 11 to 16 to think hard about covalent bonding (gcse and key stage 3) “a covalent bond is a shared pair of electrons”. My teaching philosophy is engagement through application.
Draw the full atomic structure/electronic configuration of. It will also help students make connections and differentiate between the types of bonds and have a better understanding of the nomenclature of ionic and covalent compounds. Divide students into pairs and give each pair a paper bag with the notecards inside.
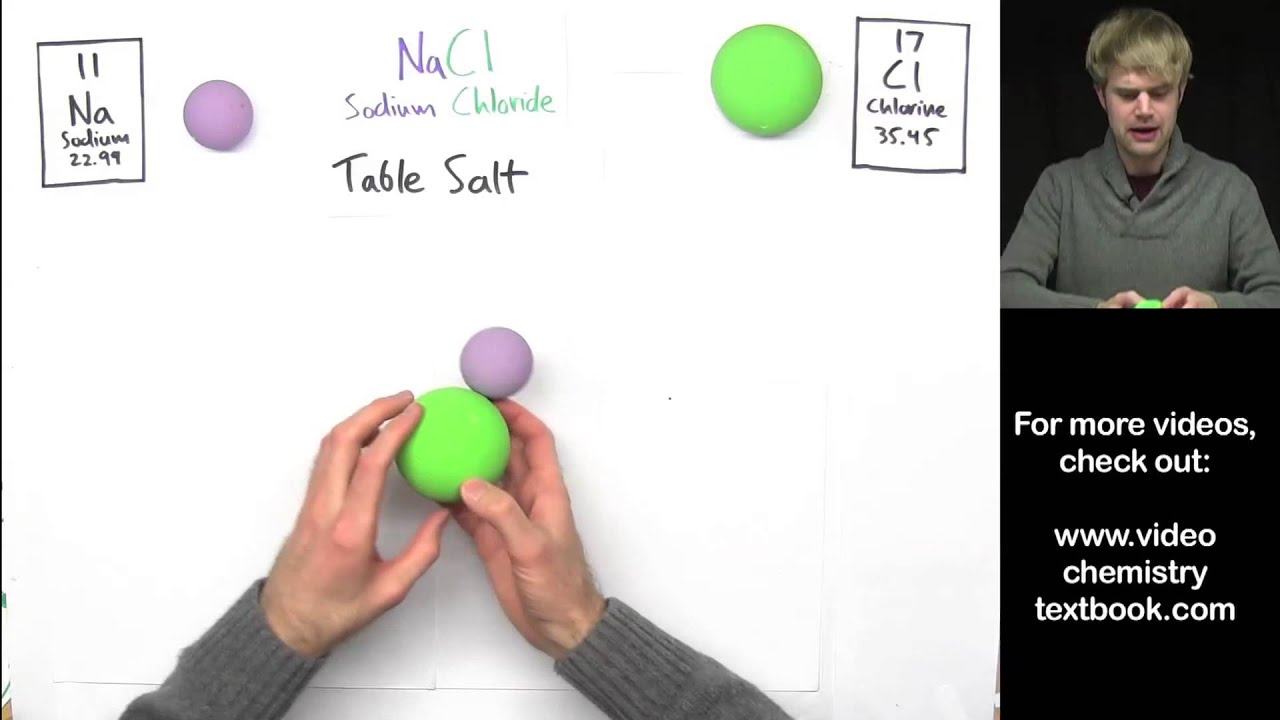
Ionic bond, also known as electrovalent bond, is a type of bond formed from the electrostatic attraction between opposit 1th, 2022chem1001 worksheet 3:. Chemistry educators at high schools need to stress on the importance of deep learning over the surface learning and engage the students in a discourse process while. Each element has its own unique atom made up of a specific number of protons in its nucleus called the.
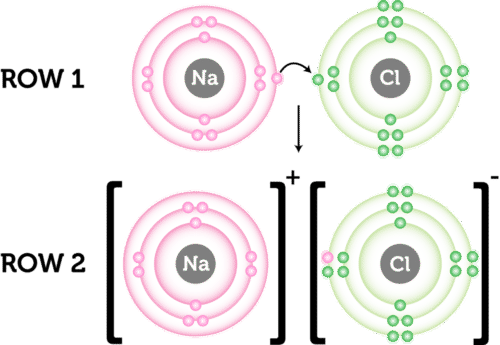
Use one of our ionic bonding “bracket” activities to help students demonstrate their understanding of ionic bonding and ionic properties. Ions are formed when atoms lose or gain electrons to obtain the stable electron arrangement of a noble gas. The goal of this plan is to help teachers teach ions and ionic bonding based on electrostatics and.
Ionic compounds form lattice structures of oppositely. The activity, my name is bond, ionic bond. One student pulls an ionic bond from the bag and tries to get their partner to guess which ionic.
Are you students having trouble determining whether a compound is ionic or covalent? View this quick and easy strategy that students can utilize to learn th. When two or more oppositely charged ions are held together due to the presence of electrostatic force, the resulting bond is termed an ionic bond.